|
Other articles:
|
In the introduction to chemical kinetics, we have already defined chemical reaction rates. Rates of chemical reactions depend on the nature of the reactants .

 Feb 27, 2011 . I cannot figure out how to find the production rate of a chemical reaction. We are given the equation : CH3Br + OH- --> CH3OH + Br- followed .
Feb 27, 2011 . I cannot figure out how to find the production rate of a chemical reaction. We are given the equation : CH3Br + OH- --> CH3OH + Br- followed .
 Nov 4, 2004 . In order to understand how the concentrations of the species in a chemical reaction change with time it is necessary to integrate the rate .
File Format: PDF/Adobe Acrobat - Quick View
File Format: PDF/Adobe Acrobat
Rates of Chemical Reactions I: A Clock Reaction.
Nov 4, 2004 . In order to understand how the concentrations of the species in a chemical reaction change with time it is necessary to integrate the rate .
File Format: PDF/Adobe Acrobat - Quick View
File Format: PDF/Adobe Acrobat
Rates of Chemical Reactions I: A Clock Reaction.


 Oct 4, 2007 . An overview of the important discipline of kinetics - the study of how fast chemical reactions go, what conditions affect these rates and .
Jump to Catalysts and the Rates of Chemical Reactions: A small quantity of catalyst should be able to affect the rate of reaction for a large amount of .
The rate of change in the concentrations of the reactants and products can be used to characterize the rate of a chemical reaction. The rate of change in .
Oct 4, 2007 . An overview of the important discipline of kinetics - the study of how fast chemical reactions go, what conditions affect these rates and .
Jump to Catalysts and the Rates of Chemical Reactions: A small quantity of catalyst should be able to affect the rate of reaction for a large amount of .
The rate of change in the concentrations of the reactants and products can be used to characterize the rate of a chemical reaction. The rate of change in .


 Iron rusting - a chemical reaction with a slow reaction rate. . . For a chemical reaction n A + m B → C + D, the rate equation or rate law is a .
Catalysts: catalysts are substances that change the rate of a chemical reaction without being changed in the reaction. Catalysts are most often used to .
Iron rusting - a chemical reaction with a slow reaction rate. . . For a chemical reaction n A + m B → C + D, the rate equation or rate law is a .
Catalysts: catalysts are substances that change the rate of a chemical reaction without being changed in the reaction. Catalysts are most often used to .
 A catalyst is a substance that alters the rate of a chemical reaction without being used up or permanently changed chemically. .
Nov 10, 2006 . Catalysts speed up chemical reactions. Only very minute quantities of the catalyst are required to produce a dramatic change in the rate of .
The central theme to be developed in this chapter is that the rate of a chemical reaction depends on its reaction mechanism . Two molecules coming together .
These enzyme molecules are organic catalysts; they speed up the rates of the chemical conversion process. None of the chemical reactions in living things .
The rate of a reaction is the speed at which a reaction happens. If a reaction has a low . Concentration, Temperature, and Pressure change reaction rates .
Factors that Affect the Chemical Reaction Rate - Reaction Kinetics · Zero Order Reaction Definition - Chemistry Glossary Definition of Zero Orde. .
Which of the following methods for increasing the rate of chemical reaction increases the effectiveness of the interactions between reactants, instead of .
A catalyst is a substance that alters the rate of a chemical reaction without being used up or permanently changed chemically. .
Nov 10, 2006 . Catalysts speed up chemical reactions. Only very minute quantities of the catalyst are required to produce a dramatic change in the rate of .
The central theme to be developed in this chapter is that the rate of a chemical reaction depends on its reaction mechanism . Two molecules coming together .
These enzyme molecules are organic catalysts; they speed up the rates of the chemical conversion process. None of the chemical reactions in living things .
The rate of a reaction is the speed at which a reaction happens. If a reaction has a low . Concentration, Temperature, and Pressure change reaction rates .
Factors that Affect the Chemical Reaction Rate - Reaction Kinetics · Zero Order Reaction Definition - Chemistry Glossary Definition of Zero Orde. .
Which of the following methods for increasing the rate of chemical reaction increases the effectiveness of the interactions between reactants, instead of .

 File Format: PDF/Adobe Acrobat - Quick View
1 answer - Apr 21When the concentration of a substance is doubled what effect does it . concentrations have noo effect but rates do effects affinity to react. .
Several factors affect the rate at which chemical reactions proceed. Understanding these factors will help you predict the direction and speed of a chemical .
File Format: Microsoft Powerpoint - Quick View
Surface area and rate of chemical reaction 2 min - Apr 13, 2008 - Uploaded by kosasihiskandarsjah
By running a chemical reaction under varying conditions, you will explore this study question: What influences the rate of a chemical reaction? .
File Format: PDF/Adobe Acrobat - Quick View
File Format: PDF/Adobe Acrobat - Quick View
1 answer - Apr 21When the concentration of a substance is doubled what effect does it . concentrations have noo effect but rates do effects affinity to react. .
Several factors affect the rate at which chemical reactions proceed. Understanding these factors will help you predict the direction and speed of a chemical .
File Format: Microsoft Powerpoint - Quick View
Surface area and rate of chemical reaction 2 min - Apr 13, 2008 - Uploaded by kosasihiskandarsjah
By running a chemical reaction under varying conditions, you will explore this study question: What influences the rate of a chemical reaction? .
File Format: PDF/Adobe Acrobat - Quick View
.bmp) A general approximate rule for the effect of temperature on reaction rates is that the reaction rate for most of the chemical reactions becomes almost .
Chemical Kinetics: Terms and Concepts - Intro duction to kinetics with links to sections on Extent of a Chemical Reaction, Rate of a Chemical Reaction, .
Mar 4, 2011 . The rate of a chemical reaction will depend on the concentrations of the reactants, although not always in the way that might be expected .
A general approximate rule for the effect of temperature on reaction rates is that the reaction rate for most of the chemical reactions becomes almost .
Chemical Kinetics: Terms and Concepts - Intro duction to kinetics with links to sections on Extent of a Chemical Reaction, Rate of a Chemical Reaction, .
Mar 4, 2011 . The rate of a chemical reaction will depend on the concentrations of the reactants, although not always in the way that might be expected .

 File Format: PDF/Adobe Acrobat - Quick View
File Format: PDF/Adobe Acrobat - Quick View
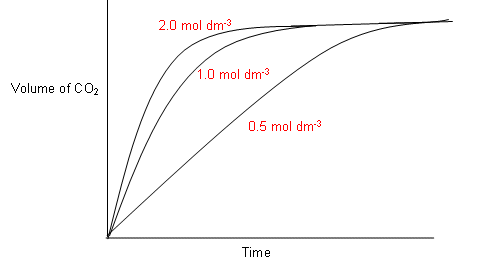
 Measurements of Reaction Rates · Factors Affecting Reaction Rates · Collision Theory of Reaction Rates · Activation Energy.
Measurements of Reaction Rates · Factors Affecting Reaction Rates · Collision Theory of Reaction Rates · Activation Energy.
 File Format: Microsoft Word - Quick View
File Format: Microsoft Word - Quick View
 Laser controls chemical reaction rates. Oct 13, 2006. Physicists in Canada are the first to use laser light as a catalyst to control chemical reactions. .
All chemical reactions have different rates of reactions. . . As previously mentioned, the rate of a chemical reaction cannot be worked out by looking at .
File Format: PDF/Adobe Acrobat - Quick View
Laser controls chemical reaction rates. Oct 13, 2006. Physicists in Canada are the first to use laser light as a catalyst to control chemical reactions. .
All chemical reactions have different rates of reactions. . . As previously mentioned, the rate of a chemical reaction cannot be worked out by looking at .
File Format: PDF/Adobe Acrobat - Quick View
 Chemistry Tutorial 9.01b: Factors Affecting Reaction Rate 8 min - Oct 1, 2009 - Uploaded by MarkRosengarten
Chemistry Tutorial 9.01b: Factors Affecting Reaction Rate 8 min - Oct 1, 2009 - Uploaded by MarkRosengarten
 Apr 2, 2005 . The rate for the reaction increases. Reaction rates are roughly doubled when the temperature increases by 10 degrees Kelvin. .
Apr 2, 2005 . The rate for the reaction increases. Reaction rates are roughly doubled when the temperature increases by 10 degrees Kelvin. .
 File Format: PDF/Adobe Acrobat - Quick View
File Format: PDF/Adobe Acrobat - Quick View
 Rates of Chemical Reactions. Synopsis. The concept of "rate" is not a new one to most people. We talk about the rate at which we type and this rate is .
Jan 13, 2011 . Section 2: Rates of Chemical Reactions. Self-Check Quiz-Eng. . The minimum amount of energy to start any chemical reaction is called the .
Rates of Chemical Reactions. Synopsis. The concept of "rate" is not a new one to most people. We talk about the rate at which we type and this rate is .
Jan 13, 2011 . Section 2: Rates of Chemical Reactions. Self-Check Quiz-Eng. . The minimum amount of energy to start any chemical reaction is called the .
 Test your knowledge on rates of chemical reactions.
Catalysts speed up chemical reactions. Only very minute quantities of the catalyst are required to produce a dramatic change in the rate of the reaction. .
File Format: Microsoft Powerpoint - Quick View
The 'rule of thumb' that the rate of chemical reactions doubles for every 10 °C temperature rise is a common misconception. This may have been generalized .
Chemistry question: What affects the rate of a chemical reaction? There is a near-infinite list of what might effect reaction rates.
File Format: PDF/Adobe Acrobat - Quick View
Test your knowledge on rates of chemical reactions.
Catalysts speed up chemical reactions. Only very minute quantities of the catalyst are required to produce a dramatic change in the rate of the reaction. .
File Format: Microsoft Powerpoint - Quick View
The 'rule of thumb' that the rate of chemical reactions doubles for every 10 °C temperature rise is a common misconception. This may have been generalized .
Chemistry question: What affects the rate of a chemical reaction? There is a near-infinite list of what might effect reaction rates.
File Format: PDF/Adobe Acrobat - Quick View
 A substance that increases the rate of a chemical reaction, without being consumed or produced by the reaction. Catalysts speed both the forward and reverse .
A substance that increases the rate of a chemical reaction, without being consumed or produced by the reaction. Catalysts speed both the forward and reverse .
 ENZYMES AND THE RATE OF CHEMICAL REACTIONS. Introduction: Enzymes are proteins that speed up the rate of a chemical reaction without being used up. .
ENZYMES AND THE RATE OF CHEMICAL REACTIONS. Introduction: Enzymes are proteins that speed up the rate of a chemical reaction without being used up. .
 13.1 Chemical Kinetics—A Preview—Chemical kinetics encompasses the measurement of reaction rates, the study of the factors affecting these rates, and the .
13.1 Chemical Kinetics—A Preview—Chemical kinetics encompasses the measurement of reaction rates, the study of the factors affecting these rates, and the .



 However, because of the relatively small diffusion rates in solids, the .
Do these "Chemical Reactions" demos and "Chemical Reactions" lab . Explore rates of reaction with the "Iodine Clock Reaction" lab . .
Sitemap
However, because of the relatively small diffusion rates in solids, the .
Do these "Chemical Reactions" demos and "Chemical Reactions" lab . Explore rates of reaction with the "Iodine Clock Reaction" lab . .
Sitemap
|












.bmp)





























.bmp)
















