|
Other articles:
|
An increase in temperature increases the rate of a chemical reaction by what means . If the intial rate of the reaction consumes 1 mole of phosphorus, P4, .
Measuring the Rate of the Reaction between. 1) Dilute Hydrochloric Acid and Calcium Carbonate. 2) Dilute Hydrochloric Acid and Sodium Thiosulfate .


 This information is especially useful for determining how a reaction occurs. What is meant by the speed of a reaction? The speed of a reaction is the rate .
This information is especially useful for determining how a reaction occurs. What is meant by the speed of a reaction? The speed of a reaction is the rate .



 Nov 10, 2006 . You might find that the rate of reaction is limited by the concentration of the weaker solution, and increasing the concentration of the .
Aug 12, 2009 . Explore what makes a reaction happen by colliding atoms and molecules. Design experiments with different reactions, concentrations, .
Nov 10, 2006 . You might find that the rate of reaction is limited by the concentration of the weaker solution, and increasing the concentration of the .
Aug 12, 2009 . Explore what makes a reaction happen by colliding atoms and molecules. Design experiments with different reactions, concentrations, .
 The rate law or rate equation for a chemical reaction is an equation that links the reaction rate with concentrations or pressures of reactants and constant .
If we measure the reaction rate just after we define the reaction conditions, . More information about the reaction rate law is needed than a single .
The rate law or rate equation for a chemical reaction is an equation that links the reaction rate with concentrations or pressures of reactants and constant .
If we measure the reaction rate just after we define the reaction conditions, . More information about the reaction rate law is needed than a single .
 Reactions between molecules are usually slower than ions. In molecules bonds have to be broken and new bonds reformed. This slows down reaction rates. .
Rate of reaction is concerned with how quickly a reaction reaches a certain point. It can be defined as the decrease in concentration of the reactants per .
Nov 4, 2004 . The study of the rate of reactions is called chemical kinetics. . In a first order reaction the rate is proportional to the concentration .
The rate of a chemical reaction is defined as the change in the concentration of one of the reactants or products in unit time .
Mar 27, 2008 . This experiment shows changes in the rate of reaction between iron(III) nitrate solution and sodium thiosulfate solution using a range of .
Reactions between molecules are usually slower than ions. In molecules bonds have to be broken and new bonds reformed. This slows down reaction rates. .
Rate of reaction is concerned with how quickly a reaction reaches a certain point. It can be defined as the decrease in concentration of the reactants per .
Nov 4, 2004 . The study of the rate of reactions is called chemical kinetics. . In a first order reaction the rate is proportional to the concentration .
The rate of a chemical reaction is defined as the change in the concentration of one of the reactants or products in unit time .
Mar 27, 2008 . This experiment shows changes in the rate of reaction between iron(III) nitrate solution and sodium thiosulfate solution using a range of .



 Pressure cookers, top right, are used to cook food at temperatures above 100 oC. Food cooks quickly at such high temperatures as the rate of reaction .
A catalyst is a substance that alters the rate of a chemical reaction without being used up or permanently changed chemically. .
Pressure cookers, top right, are used to cook food at temperatures above 100 oC. Food cooks quickly at such high temperatures as the rate of reaction .
A catalyst is a substance that alters the rate of a chemical reaction without being used up or permanently changed chemically. .

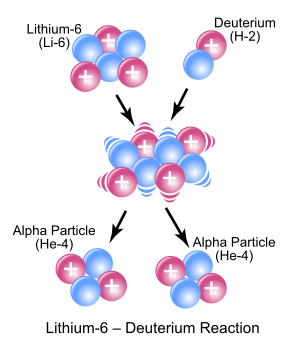 An introduction to order of reaction and rate equations.
Jul 22, 2010 . In this chemistry project, use a homemade gas collection apparatus to quantify how reactant particle size affects reaction rate when .
An introduction to order of reaction and rate equations.
Jul 22, 2010 . In this chemistry project, use a homemade gas collection apparatus to quantify how reactant particle size affects reaction rate when .

 lrc-srvr.mps.ohio-state.edu/under/chemed/qbank/quiz/bank10.htm - SimilarBBC - GCSE Bitesize: Measuring ratesThe rate of a reaction can be measured by the rate at which a reactant [reactant : substances present at the start of a chemical reaction ] is used up, .
Nov 11, 2010 . How do you assess how successful a reaction is going? The best way is to look at the speed - the rate of the reaction.
Mar 21, 2008 . What is rate of reaction? 1. Rate of reaction is defined as the change in the amount of reactants or products per unit time. 2. .
A tutorial on reaction rates or chemical kinetics suitable for high school students.
Jump to Reversible Reactions: If the rate of formation of A for the forward reaction (A . If the rate of reaction were -rA = kCACB then we would have .
lrc-srvr.mps.ohio-state.edu/under/chemed/qbank/quiz/bank10.htm - SimilarBBC - GCSE Bitesize: Measuring ratesThe rate of a reaction can be measured by the rate at which a reactant [reactant : substances present at the start of a chemical reaction ] is used up, .
Nov 11, 2010 . How do you assess how successful a reaction is going? The best way is to look at the speed - the rate of the reaction.
Mar 21, 2008 . What is rate of reaction? 1. Rate of reaction is defined as the change in the amount of reactants or products per unit time. 2. .
A tutorial on reaction rates or chemical kinetics suitable for high school students.
Jump to Reversible Reactions: If the rate of formation of A for the forward reaction (A . If the rate of reaction were -rA = kCACB then we would have .



 Purpose The purpose of this investigation is to determine the effect that varying temperatures have on the rate of a reaction. Introduction Based on the .
Purpose The purpose of this investigation is to determine the effect that varying temperatures have on the rate of a reaction. Introduction Based on the .
.bmp) A searchable database of terms about Reaction rates for students and teachers of general chemistry.
File Format: PDF/Adobe Acrobat - Quick View
The line has the classic shape of a rate of reaction graph. It starts off steep, becoming shallower until it levels off. You can tell the rate of reaction .
Chemistry Music Video 22: Rate Of Reaction 3 min - Aug 30, 2009 - Uploaded by MarkRosengarten
A chemist who investigates the rate of a reaction often wants to see how the rate differs at two different temperatures. In the video below, this situation .
Science question: How does temperature affect the rate of reaction? Higher temperature increases rate For the very large majority of chemical reactions, .
Apr 2, 2005 . Counting by weighing and the mole are explained. Avogadro's number is explained. Examples are shown for calculaing molar mass.
A searchable database of terms about Reaction rates for students and teachers of general chemistry.
File Format: PDF/Adobe Acrobat - Quick View
The line has the classic shape of a rate of reaction graph. It starts off steep, becoming shallower until it levels off. You can tell the rate of reaction .
Chemistry Music Video 22: Rate Of Reaction 3 min - Aug 30, 2009 - Uploaded by MarkRosengarten
A chemist who investigates the rate of a reaction often wants to see how the rate differs at two different temperatures. In the video below, this situation .
Science question: How does temperature affect the rate of reaction? Higher temperature increases rate For the very large majority of chemical reactions, .
Apr 2, 2005 . Counting by weighing and the mole are explained. Avogadro's number is explained. Examples are shown for calculaing molar mass.
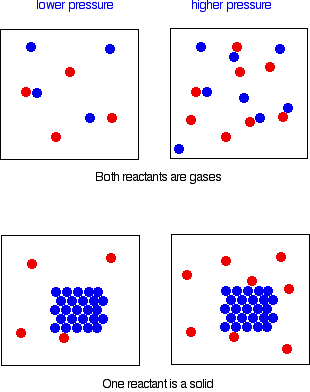 File Format: PDF/Adobe Acrobat - Quick View
The rate of reaction, r, is defined to be the slope of the concentration-time plot for a species divided by the stoichiometric coefficient of that species. .
Dec 29, 2008 . The Rates of Reaction Crossword. How do I play? It is really easy to play, unless you don't know the answers! The grid is not numbered .
The rate of a reaction is the speed at which a reaction happens. . There is another big idea for rates of reaction called collision theory. .
May 6, 2011 . Refer to any previous laboratory experience with 'rate of reaction' experiments which may have helped you decide and design the experimental .
A summary of Determining the Rate Law in 's Reaction Kinetics: Rate Laws. Learn exactly what happened in this chapter, scene, or section of Reaction .
Concentration and Reaction Rates - Science Theater 10 5 min - Sep 21, 2008 - Uploaded by sciencetheater
Several factors affect the rate at which chemical reactions proceed. Understanding these factors will help you predict the direction and speed of a chemical .
The reaction rate(rate of reaction) or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a .
The rate of a reaction depends on the rate of the slowest step in its mechanism. . In mixture 4 in the Rate of Reaction lab, you diluted 5.0 mL of 0.20 M .
4 posts - 3 authors - Last post: Mar 16I believe this is a valid way to do it as you generally measure rate of reaction as the change in the concentration of some reactant or .
This page describes the factors controlling the speeds of chemical reactions and the collision theory behind it discussed. The factors affecting the speed .
Jump to The initial rate of reaction.: The initial rate of a reaction is the instantaneous rate at the start of the reaction (i.e., when t = 0). .
By rate of reaction we mean the change in concentration of a reactant (or a product) in a given period of time. This might, for example, be over a short .
File Format: Microsoft Powerpoint - Quick View
File Format: PDF/Adobe Acrobat - Quick View
The rate of reaction, r, is defined to be the slope of the concentration-time plot for a species divided by the stoichiometric coefficient of that species. .
Dec 29, 2008 . The Rates of Reaction Crossword. How do I play? It is really easy to play, unless you don't know the answers! The grid is not numbered .
The rate of a reaction is the speed at which a reaction happens. . There is another big idea for rates of reaction called collision theory. .
May 6, 2011 . Refer to any previous laboratory experience with 'rate of reaction' experiments which may have helped you decide and design the experimental .
A summary of Determining the Rate Law in 's Reaction Kinetics: Rate Laws. Learn exactly what happened in this chapter, scene, or section of Reaction .
Concentration and Reaction Rates - Science Theater 10 5 min - Sep 21, 2008 - Uploaded by sciencetheater
Several factors affect the rate at which chemical reactions proceed. Understanding these factors will help you predict the direction and speed of a chemical .
The reaction rate(rate of reaction) or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a .
The rate of a reaction depends on the rate of the slowest step in its mechanism. . In mixture 4 in the Rate of Reaction lab, you diluted 5.0 mL of 0.20 M .
4 posts - 3 authors - Last post: Mar 16I believe this is a valid way to do it as you generally measure rate of reaction as the change in the concentration of some reactant or .
This page describes the factors controlling the speeds of chemical reactions and the collision theory behind it discussed. The factors affecting the speed .
Jump to The initial rate of reaction.: The initial rate of a reaction is the instantaneous rate at the start of the reaction (i.e., when t = 0). .
By rate of reaction we mean the change in concentration of a reactant (or a product) in a given period of time. This might, for example, be over a short .
File Format: Microsoft Powerpoint - Quick View
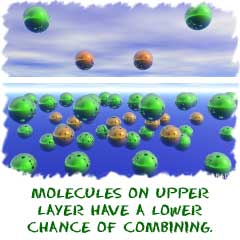 The central theme to be developed in this chapter is that the rate of a chemical reaction depends on its reaction mechanism . Two molecules coming together .
File Format: Microsoft Powerpoint - View as HTML
In this coursework I plan to investigate the effect of varying the temperature of sodium thiosulphate on the rate of reaction between dilute sodium .
The central theme to be developed in this chapter is that the rate of a chemical reaction depends on its reaction mechanism . Two molecules coming together .
File Format: Microsoft Powerpoint - View as HTML
In this coursework I plan to investigate the effect of varying the temperature of sodium thiosulphate on the rate of reaction between dilute sodium .
 Discusses the collision theory of reaction rates, including the importance of activation energy and the Maxwell-Boltzmann distribution. .
Dec 22, 2010 . In such a case the rate of reaction differs from the rate of increase of concentration of a product P by a constant factor (the reciprocal .
Discusses the collision theory of reaction rates, including the importance of activation energy and the Maxwell-Boltzmann distribution. .
Dec 22, 2010 . In such a case the rate of reaction differs from the rate of increase of concentration of a product P by a constant factor (the reciprocal .
 Sitemap
Sitemap
|













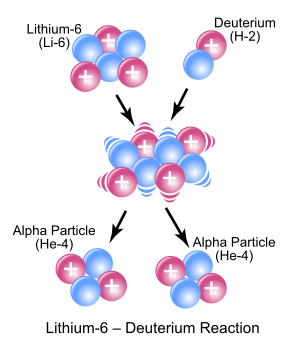






.bmp)























.bmp)



