|
Other articles:
|
VSEPR: Valence Shell Electron Pair Repulsion Theory. Valence Shell Electron Pair Repulsion Theory, or VSEPR for short, is a model used to predcit the .
 Using VSEPR; Rules · Counting Regions of High Electron Density · Practice Problems . Molecular Structures Based on VSEPR Theory .
File Format: PDF/Adobe Acrobat - Quick View
VSEPR Tutorial by Brenda Wojciechowski and Paul Cerpovicz at Georgia Southern . VSEPR Tables by Fei Liu and Marcus Horne at Lebanon Valley College .
Feb 17, 2010 . VSEPR Analysis of Inorganic Triatomic Molecules and Ions? - clf2- vsepr 1. How many pairs of electrons on the central atom of the following? .
VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of atoms about a central atom in a covalent compound, or charged ion, .
Valence shell electron pair repulsion (VSEPR) model.
Sometimes people have a hard time with the whole VSEPR thing. In this helpdesk section we'll discuss what VSEPR means, what it's all about, and how you can .
VSEPR theory - Description: Valence shell electron pair repulsion (VSEPR) theory is a model in chemistry used to predict the shape of individual molecules .
Molecular geometry or shape is heavily influenced by valence shell electron pair repulsion. Choose from 4 VSEPR theory molecular models to make illustrating .
Valence shell electron pair repulsion (VSEPR) theory is a model in chemistry used to predict the shape of individual molecules based upon the extent of .
Using VSEPR; Rules · Counting Regions of High Electron Density · Practice Problems . Molecular Structures Based on VSEPR Theory .
File Format: PDF/Adobe Acrobat - Quick View
VSEPR Tutorial by Brenda Wojciechowski and Paul Cerpovicz at Georgia Southern . VSEPR Tables by Fei Liu and Marcus Horne at Lebanon Valley College .
Feb 17, 2010 . VSEPR Analysis of Inorganic Triatomic Molecules and Ions? - clf2- vsepr 1. How many pairs of electrons on the central atom of the following? .
VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of atoms about a central atom in a covalent compound, or charged ion, .
Valence shell electron pair repulsion (VSEPR) model.
Sometimes people have a hard time with the whole VSEPR thing. In this helpdesk section we'll discuss what VSEPR means, what it's all about, and how you can .
VSEPR theory - Description: Valence shell electron pair repulsion (VSEPR) theory is a model in chemistry used to predict the shape of individual molecules .
Molecular geometry or shape is heavily influenced by valence shell electron pair repulsion. Choose from 4 VSEPR theory molecular models to make illustrating .
Valence shell electron pair repulsion (VSEPR) theory is a model in chemistry used to predict the shape of individual molecules based upon the extent of .
 Oct 2, 2000 . Valence Shell Electron Pair Repulsion (VSEPR) theory allows the Chemist to predict the 3-dimensional shape of molecules from knowledge of .
May 10, 2002 . Page for Professor Winn's Chemistry 6 (9 AM) course.
Oct 2, 2000 . Valence Shell Electron Pair Repulsion (VSEPR) theory allows the Chemist to predict the 3-dimensional shape of molecules from knowledge of .
May 10, 2002 . Page for Professor Winn's Chemistry 6 (9 AM) course.
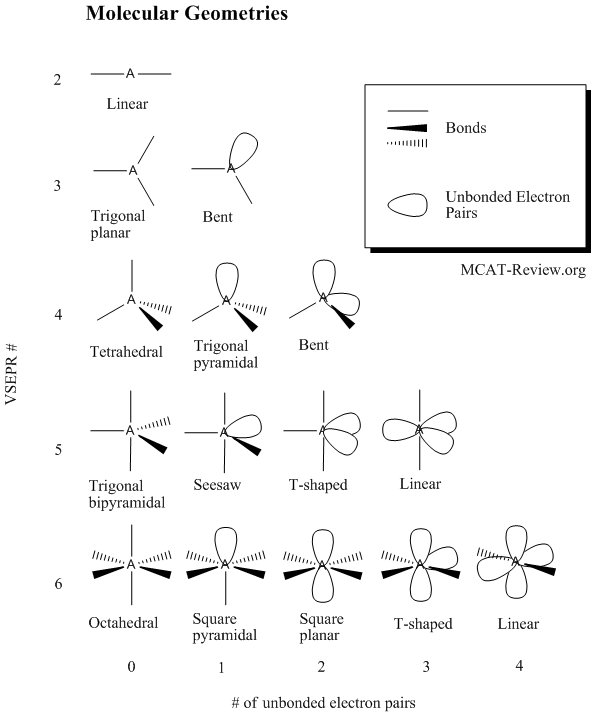
 Jul 19, 2006 . The Valence Shell Electron Pair Repulsion Theory (VSEPR), as it is traditionally called helps us to understand the 3d structure of molecules .
Science, Science-Chemistry, resonance isomers, dot vsepr, expanded octet, nh, sentation lewis, bonding pair, chem, bonding vsepr, polarity diagram, .
∨ヤ「 ∵ネム > チ ≠ァ ⊆ネム ネム ⇒ ′策 ¶頃 〒 ! ゚ ∩カェ ナ Åホ ? 9 = &* ホニム 「 ヨ 享 .
Jul 19, 2006 . The Valence Shell Electron Pair Repulsion Theory (VSEPR), as it is traditionally called helps us to understand the 3d structure of molecules .
Science, Science-Chemistry, resonance isomers, dot vsepr, expanded octet, nh, sentation lewis, bonding pair, chem, bonding vsepr, polarity diagram, .
∨ヤ「 ∵ネム > チ ≠ァ ⊆ネム ネム ⇒ ′策 ¶頃 〒 ! ゚ ∩カェ ナ Åホ ? 9 = &* ホニム 「 ヨ 享 .
 Welcome to this introduction to VSEPR rules for the prediction of molecular shape. In addition, try the Sheffield Chemputer one component of which is an .
Valance Shell Electron. Pair Repulsion. (VSEPR). An Interacive Tutorial & Quiz. Enter. or back to the Virtual Laboratory.
File Format: PDF/Adobe Acrobat - Quick View
An introduction to VSEPR theory. Includes links to pages with example PDB files which may be viewed using Chemscape Chime.
Welcome to this introduction to VSEPR rules for the prediction of molecular shape. In addition, try the Sheffield Chemputer one component of which is an .
Valance Shell Electron. Pair Repulsion. (VSEPR). An Interacive Tutorial & Quiz. Enter. or back to the Virtual Laboratory.
File Format: PDF/Adobe Acrobat - Quick View
An introduction to VSEPR theory. Includes links to pages with example PDB files which may be viewed using Chemscape Chime.
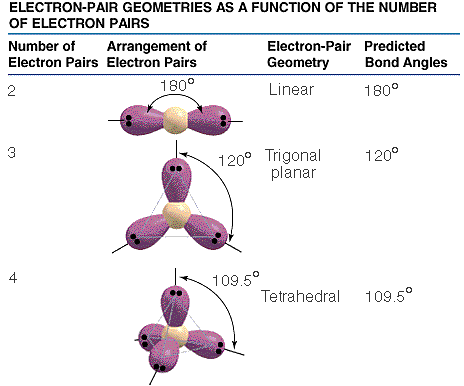
 Loading. please wait.
Loading. please wait.
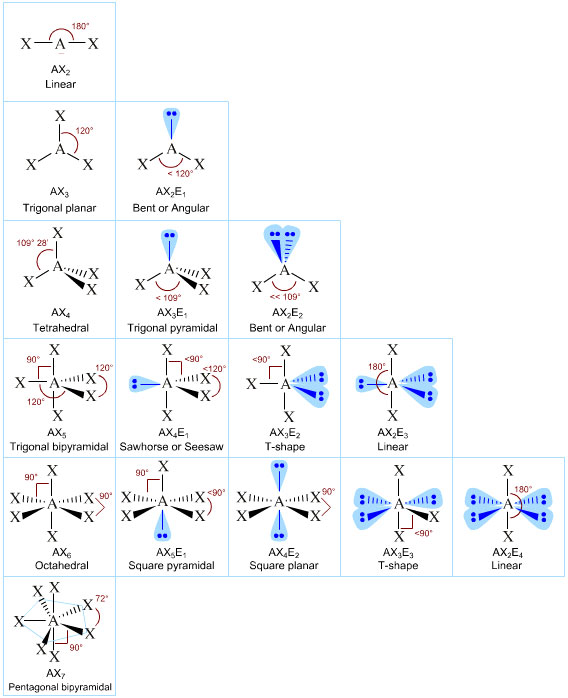
 The VSEPR method of predicting molecular shapes, allows one to get from a formula of say PF5 to the three dimensional structure at the left. .
. is to use a simple approach that builds on the Lewis-Dot structure approach called the Valence Shell Electron Pair Repulsion Model, or VSEPR model. .
File Format: PDF/Adobe Acrobat - Quick View
We will use a model called the Valence Shell Electron-Pair Repulsion (VSEPR) model that is based on the repulsive behavior of electron-pairs. .
File Format: PDF/Adobe Acrobat - Quick View
The basis of the VSEPR theory is the fact that pairs of valence electrons in bonded atoms repel one another. This repulsion pushes electron pairs as far .
This tutorial visually summarizes the various geometries described by VSEPR theory. Structures of the different types of shapes and molecules that serve as .
File Format: Shockwave Flash
Valence-shell electron-pair repulsion (VSEPR) theory . . Colorful charts of Lewis Structures compared to their respective VSEPR molecular shapes. .
Carbon Dioxide The ideal O-C-O bond angle is 180o. The experimental O-C-O bond angle is 180o. Ozone The ideal O-O-O bond angle is 120o .
Jul 21, 2009 . Valence shell electron pair repulsion (VSEPR) theory (1957) is a model in chemistry, which is used for predicting the shapes of individual .
The VSEPR method of predicting molecular shapes, allows one to get from a formula of say PF5 to the three dimensional structure at the left. .
. is to use a simple approach that builds on the Lewis-Dot structure approach called the Valence Shell Electron Pair Repulsion Model, or VSEPR model. .
File Format: PDF/Adobe Acrobat - Quick View
We will use a model called the Valence Shell Electron-Pair Repulsion (VSEPR) model that is based on the repulsive behavior of electron-pairs. .
File Format: PDF/Adobe Acrobat - Quick View
The basis of the VSEPR theory is the fact that pairs of valence electrons in bonded atoms repel one another. This repulsion pushes electron pairs as far .
This tutorial visually summarizes the various geometries described by VSEPR theory. Structures of the different types of shapes and molecules that serve as .
File Format: Shockwave Flash
Valence-shell electron-pair repulsion (VSEPR) theory . . Colorful charts of Lewis Structures compared to their respective VSEPR molecular shapes. .
Carbon Dioxide The ideal O-C-O bond angle is 180o. The experimental O-C-O bond angle is 180o. Ozone The ideal O-O-O bond angle is 120o .
Jul 21, 2009 . Valence shell electron pair repulsion (VSEPR) theory (1957) is a model in chemistry, which is used for predicting the shapes of individual .
 VSEPR. Copyright 2000 by James P. Birk. If you do not see an image with a black background here, you must download and install the free Chime plugin. .
Jul 20, 2010 . An at home molecular modeling experiment that uses paper models and Pladoh ball and stick models to illustrate the theory behind molecular .
File Format: PDF/Adobe Acrobat - Quick View
Tutorial. Molecular Shapes. Examples. Problems · Back VR Lab. Use the arrows for navigation!
undergrad-ed.chemistry.ohio-state.edu/VSEPR/ - SimilarVSEPR modelThe VSEPR (Valance Shell Electron Pair Repulsion) theory is based on the principle that electron pairs around an atom repel each other. .
Jun 1, 1998 . VSEPR Theory is one method that chemists use to predict the shapes of molecules. This theory predicts that electron pairs, whether involved .
File Format: PDF/Adobe Acrobat - View as HTML
Valence shell electron pair repulsion, VSEPR, is a super-simple technique for predicting the geometry of atomic centres in small molecules and molecular .
VSEPR QUIZ 1. Quiz 1. Select the best answer from the choices provided. Show all questions. <= =>. What is the molecular geometry around the central atom in .
The VSEPR theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that .
VSEPR. Copyright 2000 by James P. Birk. If you do not see an image with a black background here, you must download and install the free Chime plugin. .
Jul 20, 2010 . An at home molecular modeling experiment that uses paper models and Pladoh ball and stick models to illustrate the theory behind molecular .
File Format: PDF/Adobe Acrobat - Quick View
Tutorial. Molecular Shapes. Examples. Problems · Back VR Lab. Use the arrows for navigation!
undergrad-ed.chemistry.ohio-state.edu/VSEPR/ - SimilarVSEPR modelThe VSEPR (Valance Shell Electron Pair Repulsion) theory is based on the principle that electron pairs around an atom repel each other. .
Jun 1, 1998 . VSEPR Theory is one method that chemists use to predict the shapes of molecules. This theory predicts that electron pairs, whether involved .
File Format: PDF/Adobe Acrobat - View as HTML
Valence shell electron pair repulsion, VSEPR, is a super-simple technique for predicting the geometry of atomic centres in small molecules and molecular .
VSEPR QUIZ 1. Quiz 1. Select the best answer from the choices provided. Show all questions. <= =>. What is the molecular geometry around the central atom in .
The VSEPR theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that .
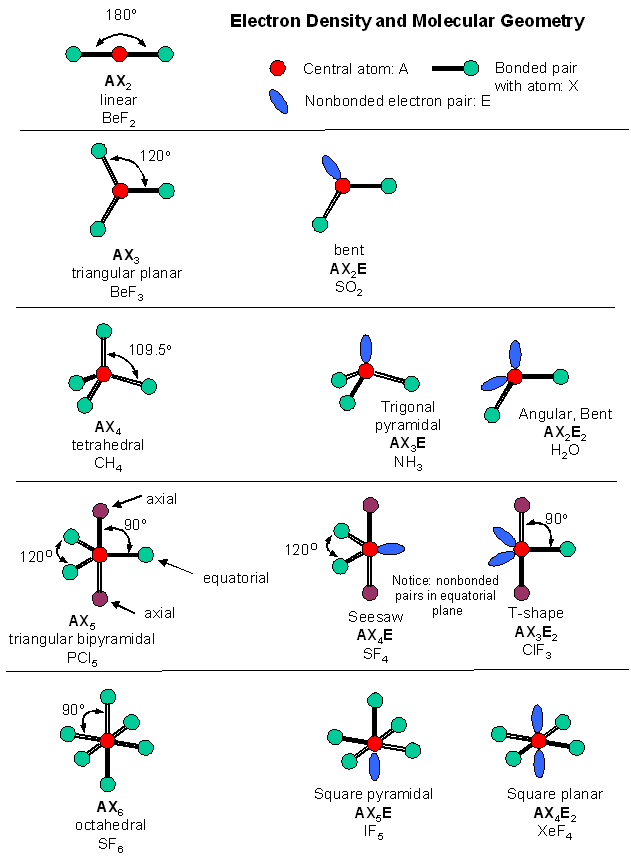 Valence shell electron pair repulsion theory, VSEPR, is a super-simple technique for predicting the shape or geometry of atomic centres in small molecules .
This theory is known as the VSEPR theory, which is an acronym for the valence shell electron pair repulsion theory. It is useful for estimating the shapes .
VSEPR Theory- The valence-shell electronic-pair repulsion (VSEPR) model is an approach of using the number of electrons surrounding a central atom to study .
Chemistry VSEPR Theory 3 min - May 12, 2009 - Uploaded by Starjester1
Jun 15, 2006 . NOTE: VSEPR is also known as Electron Domain Theory . The VSEPR model gives simple rules to predict molecular shape. .
Jobs & Education question: What is the VSEPR theory? The VSEPR theory, sometimes pronounced 'vesper', stands for Valence Shell Electron Pair Repulsion.
In English and French. Lesson with diagrams and pictures.
Sitemap
Valence shell electron pair repulsion theory, VSEPR, is a super-simple technique for predicting the shape or geometry of atomic centres in small molecules .
This theory is known as the VSEPR theory, which is an acronym for the valence shell electron pair repulsion theory. It is useful for estimating the shapes .
VSEPR Theory- The valence-shell electronic-pair repulsion (VSEPR) model is an approach of using the number of electrons surrounding a central atom to study .
Chemistry VSEPR Theory 3 min - May 12, 2009 - Uploaded by Starjester1
Jun 15, 2006 . NOTE: VSEPR is also known as Electron Domain Theory . The VSEPR model gives simple rules to predict molecular shape. .
Jobs & Education question: What is the VSEPR theory? The VSEPR theory, sometimes pronounced 'vesper', stands for Valence Shell Electron Pair Repulsion.
In English and French. Lesson with diagrams and pictures.
Sitemap
|





















