|
Other articles:
|
The students will identify some physical changes of matter. 2. The students will identify some chemical changes of matter. 3. The students will distinguish .
Determine if each is a physical or chemical change.
 In a physical change, the substances are not altered chemically, but merely changed to another phase (i.e. gas, liquid, solid) or separated or combined. .
Find out what chemical and physical changes are, get examples, and learn how to tell them apart.
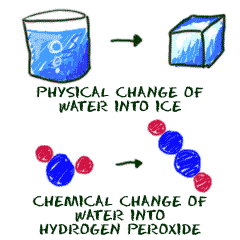
For example, the freezing of water would be a physical change because it can be . A physical change is a change in which no new substance is formed; .
File Format: PDF/Adobe Acrobat - Quick View
sparkle Physical and Chemical Change sparkle. Gamequarium Home · Science Home Last updated 09/17/2006. Line of Fish 300+ Education Sites! .
In a physical change, the substances are not altered chemically, but merely changed to another phase (i.e. gas, liquid, solid) or separated or combined. .
Find out what chemical and physical changes are, get examples, and learn how to tell them apart.
For example, the freezing of water would be a physical change because it can be . A physical change is a change in which no new substance is formed; .
File Format: PDF/Adobe Acrobat - Quick View
sparkle Physical and Chemical Change sparkle. Gamequarium Home · Science Home Last updated 09/17/2006. Line of Fish 300+ Education Sites! .

 Chemical & Physical Change. Hands-On Activities. Acids and Bases · Chemical Reactions · Dissolving · Slime and Goo. Test Your Knowledge. Chemical Reactions .
Chemical & Physical Change. Hands-On Activities. Acids and Bases · Chemical Reactions · Dissolving · Slime and Goo. Test Your Knowledge. Chemical Reactions .

 May 6, 2008 . Added to queue Chemical and Physical changeby abdou1995Featured . Added to queue Physical and Chemical Changesby millerford16742 views .
In a physical change there is only a change of state. The new substance has the same properties as the old one. No new substance(s) are produced. .
A common physical change occurs when matter changes from one phase to another. When an ice cube melts for example, it becomes liquid water. .
File Format: PDF/Adobe Acrobat - Quick View
May 6, 2008 . Added to queue Chemical and Physical changeby abdou1995Featured . Added to queue Physical and Chemical Changesby millerford16742 views .
In a physical change there is only a change of state. The new substance has the same properties as the old one. No new substance(s) are produced. .
A common physical change occurs when matter changes from one phase to another. When an ice cube melts for example, it becomes liquid water. .
File Format: PDF/Adobe Acrobat - Quick View
 Many high-school chemistry texts, general science texts, and similar authorities emphasize the distinction between “chemical” change and “physical” change. .
Many high-school chemistry texts, general science texts, and similar authorities emphasize the distinction between “chemical” change and “physical” change. .
 A physical change takes place without any changes in molecular composition. The same element or compound is present before and after the change. .
How can the two types of changes be distinguished? What are the signs of a chemical change? What are the signs of a physical change? .
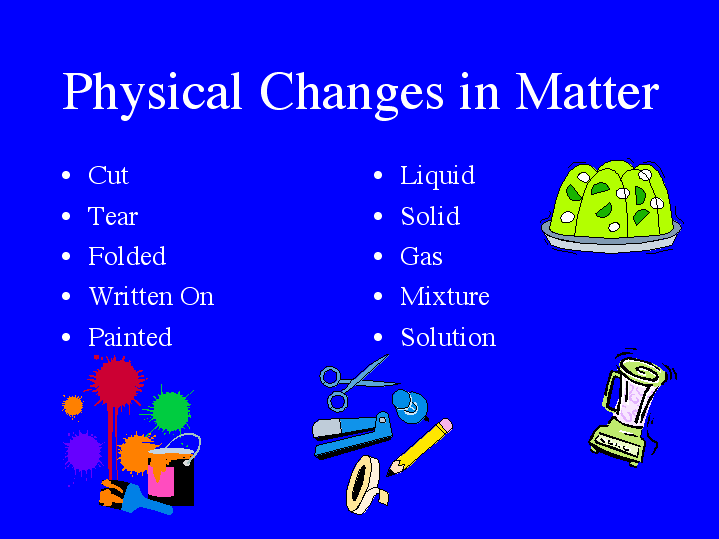
Physical change: Matter exists in three basic physical forms - solid, liquid and gas which can easily change fron one physical state to another.
A short exploration of physical changes in matter vs chemical changes in matter.
What is the difference between a physical and chemical change?
Matter is constantly experiencing both chemical and physical changes. . Physical property changes could include a change in: texture, shape, size, color , .
A physical change takes place without any changes in molecular composition. The same element or compound is present before and after the change. .
How can the two types of changes be distinguished? What are the signs of a chemical change? What are the signs of a physical change? .
Physical change: Matter exists in three basic physical forms - solid, liquid and gas which can easily change fron one physical state to another.
A short exploration of physical changes in matter vs chemical changes in matter.
What is the difference between a physical and chemical change?
Matter is constantly experiencing both chemical and physical changes. . Physical property changes could include a change in: texture, shape, size, color , .
 The years of late childhood and early adolescence contain one of the most rapid and most dramatic periods of physical change in the human life cycle. .
Chemical and Physical Change Lab. Identify whether these lab experiments are demonstrating a . Determine if each is a physical or chemical change. .
You know how everyone says that puberty is all about raging hormones; it's kind of true. Hormones that were hibernating suddenly awaken and signal your body .
The years of late childhood and early adolescence contain one of the most rapid and most dramatic periods of physical change in the human life cycle. .
Chemical and Physical Change Lab. Identify whether these lab experiments are demonstrating a . Determine if each is a physical or chemical change. .
You know how everyone says that puberty is all about raging hormones; it's kind of true. Hormones that were hibernating suddenly awaken and signal your body .
 Dirtmeister Science Lab Challenge Question: How can you force a physical change in matter? The Dirtmeister (May). What You Do, Teacher's Guide on Matter .
Feb 7, 2008 . Physical changes are those changes that do not result in the production of a new substance. If you melt a block of ice, you still have H2O .
Dirtmeister Science Lab Challenge Question: How can you force a physical change in matter? The Dirtmeister (May). What You Do, Teacher's Guide on Matter .
Feb 7, 2008 . Physical changes are those changes that do not result in the production of a new substance. If you melt a block of ice, you still have H2O .

 Now let's expand on the distinction between chemical reactions and physical changes that was alluded to earlier. Chemical reactions involve changing the .
Now let's expand on the distinction between chemical reactions and physical changes that was alluded to earlier. Chemical reactions involve changing the .
 Write and define the terms chemical change and physical change on the board. Then have the students copy the terms into their journals. .
Physical changes occur when objects or substances undergo a change that does not change their chemical nature, contrasting with the concept of chemical .
File Format: PDF/Adobe Acrobat - Quick View
What is the difference between chemical and physical change? From a database of frequently asked questions from the Matter section of General Chemistry .
Write and define the terms chemical change and physical change on the board. Then have the students copy the terms into their journals. .
Physical changes occur when objects or substances undergo a change that does not change their chemical nature, contrasting with the concept of chemical .
File Format: PDF/Adobe Acrobat - Quick View
What is the difference between chemical and physical change? From a database of frequently asked questions from the Matter section of General Chemistry .
 3 answers - Oct 10, 2006Casually mixing iron powder with sulfur powder is a physical change. Reacting iron with sulfur to produce iron sulfide is a chemical change. .
Copy & paste this link to your blog or website to reference this page. Added to Favorites. Related Searches. Physical changes of m. Difference between ch. .
A physical change is a change in which no new substances are formed and most physical changes are reversible. Examples: changes of shape, changes of states, .
The metal grill getting hot is a physical change, the charcoal reacting with oxygen (which produces the heat) is a chemical change. .
File Format: PDF/Adobe Acrobat
Pick up a copy of Physical Change and Aging A Guide for the Helping Professions, Fifth Edition (ISBN 9780826104410) by Saxon, Sue V. / Etten, Mary Jean .
3 answers - Oct 10, 2006Casually mixing iron powder with sulfur powder is a physical change. Reacting iron with sulfur to produce iron sulfide is a chemical change. .
Copy & paste this link to your blog or website to reference this page. Added to Favorites. Related Searches. Physical changes of m. Difference between ch. .
A physical change is a change in which no new substances are formed and most physical changes are reversible. Examples: changes of shape, changes of states, .
The metal grill getting hot is a physical change, the charcoal reacting with oxygen (which produces the heat) is a chemical change. .
File Format: PDF/Adobe Acrobat
Pick up a copy of Physical Change and Aging A Guide for the Helping Professions, Fifth Edition (ISBN 9780826104410) by Saxon, Sue V. / Etten, Mary Jean .
 by S Smith - 2010 - Cited by 1 - Related articles
by S Smith - 2010 - Cited by 1 - Related articles
 Physics question: What is a physical change? Answer A change that affects the size, shape or color of a substance but does not affect its composition.
Physical changes occur when objects undergo a change that does not change . Physical and chemical changes are the two basic ways that matter can change. .
File Format: PDF/Adobe Acrobat - Quick View
A physical change in a substance doesn't change what the substance is. In a chemical change where there is a chemical reaction, a new substance is formed .
Physical changes are about energy and states of matter. Chemical changes happen . When you step on a can and crush it, you have forced a physical change. .
This book serves as an authoritative textbook and guide to the physical changes and common pathologies associated with the aging process, with special .
Physics question: What is a physical change? Answer A change that affects the size, shape or color of a substance but does not affect its composition.
Physical changes occur when objects undergo a change that does not change . Physical and chemical changes are the two basic ways that matter can change. .
File Format: PDF/Adobe Acrobat - Quick View
A physical change in a substance doesn't change what the substance is. In a chemical change where there is a chemical reaction, a new substance is formed .
Physical changes are about energy and states of matter. Chemical changes happen . When you step on a can and crush it, you have forced a physical change. .
This book serves as an authoritative textbook and guide to the physical changes and common pathologies associated with the aging process, with special .

 Noun, 1. physical change - a change from one state (solid or liquid or gas) to another without a change in chemical composition .
Noun, 1. physical change - a change from one state (solid or liquid or gas) to another without a change in chemical composition .
 File Format: PDF/Adobe Acrobat - Quick View
File Format: PDF/Adobe Acrobat - Quick View

 "physical change." Encyclopædia Britannica. Encyclopædia Britannica Online. . Quick Facts. The following are quick facts associated with "physical change" .
Physical changes occur when objects undergo a change that does not change their chemical nature. A physical change involves a change in physical properties. .
Students view examples of chemical and physical change and are asked to . The Chemical and Physical Change Lab should be used after presentation of the .
File Format: Microsoft Word - Quick View
Physical change: Although some extensive properties (like shape, phase, etc.) of the material change, the material itself is the same before and after the .
"physical change." Encyclopædia Britannica. Encyclopædia Britannica Online. . Quick Facts. The following are quick facts associated with "physical change" .
Physical changes occur when objects undergo a change that does not change their chemical nature. A physical change involves a change in physical properties. .
Students view examples of chemical and physical change and are asked to . The Chemical and Physical Change Lab should be used after presentation of the .
File Format: Microsoft Word - Quick View
Physical change: Although some extensive properties (like shape, phase, etc.) of the material change, the material itself is the same before and after the .
 Sitemap
Sitemap
|







































