|
Other articles:
|
File Format: PDF/Adobe Acrobat - Quick View
Write the formula for each of the acids listed below: Nitric acid. Hydrocyanic acid. Chloric acid. Acetic acid. Hydrobromic acid. Sulfurous acid .
 Naming Acids, Oxyacids and Their Salts. If the anion does not contain oxygen, then the acid is named with the prefix hydro- and the suffix -ic. .
File Format: Microsoft Powerpoint - Quick View
If the compound starts with H, it is an acid. Use the naming acids rules. If the compound starts with C and contains quite a few H's and perhaps some O's, .
Naming Acids, Oxyacids and Their Salts. If the anion does not contain oxygen, then the acid is named with the prefix hydro- and the suffix -ic. .
File Format: Microsoft Powerpoint - Quick View
If the compound starts with H, it is an acid. Use the naming acids rules. If the compound starts with C and contains quite a few H's and perhaps some O's, .
 For the purposes of naming acids and bases we will put these chemicals into three categories: binary acids, ternary acids (sometimes called oxy-acids), .
For the purposes of naming acids and bases we will put these chemicals into three categories: binary acids, ternary acids (sometimes called oxy-acids), .

 File Format: PDF/Adobe Acrobat - Quick View
File Format: PDF/Adobe Acrobat - Quick View
 Mar 4, 2010 . @SuperTakeoff I made a separate video for acids because they have their own rules. Bases follow normal naming rules, so you can find them .
6 posts - 3 authors - Last post: Mar 5But then why is HCl called hydrochloric acid, not just chloric acid? (i mean, we do add the hydrogen for acid) .
File Format: PDF/Adobe Acrobat - Quick View
Acids are substances formed when nonmetal ions combine with hydrogen. The hydrogen is written first in the compound and the naming is based on whether the .
File Format: Microsoft Powerpoint - Quick View
In order to have some way of communicating about chemistry and chemicals it is important that we have a system of nomenclature or naming. .
Mar 4, 2010 . @SuperTakeoff I made a separate video for acids because they have their own rules. Bases follow normal naming rules, so you can find them .
6 posts - 3 authors - Last post: Mar 5But then why is HCl called hydrochloric acid, not just chloric acid? (i mean, we do add the hydrogen for acid) .
File Format: PDF/Adobe Acrobat - Quick View
Acids are substances formed when nonmetal ions combine with hydrogen. The hydrogen is written first in the compound and the naming is based on whether the .
File Format: Microsoft Powerpoint - Quick View
In order to have some way of communicating about chemistry and chemicals it is important that we have a system of nomenclature or naming. .
 Naming Acids. In order to explain acid naming, the sequence of HCl, HClO, HClO2, HClO3, and HClO4 will be discussed in order. HCl is a binary acid. .
File Format: Microsoft Powerpoint - Quick View
Naming Acids. In order to explain acid naming, the sequence of HCl, HClO, HClO2, HClO3, and HClO4 will be discussed in order. HCl is a binary acid. .
File Format: Microsoft Powerpoint - Quick View


 In this activity you will practice naming acids from thier formulas.
Jump to Naming acids: Acids are named by the anion they form when dissolved in water. If an acid forms an anion ending in ide, then its name is formed .
Naming Worksheets. These worksheets are in *.pdf format or as Microsoft Word files. . Naming acids and bases worksheet: Pretty much what it sounds like! .
Here are the names and formulas of some of the common acids and bases.
File Format: PDF/Adobe Acrobat - Quick View
In this activity you will practice naming acids from thier formulas.
Jump to Naming acids: Acids are named by the anion they form when dissolved in water. If an acid forms an anion ending in ide, then its name is formed .
Naming Worksheets. These worksheets are in *.pdf format or as Microsoft Word files. . Naming acids and bases worksheet: Pretty much what it sounds like! .
Here are the names and formulas of some of the common acids and bases.
File Format: PDF/Adobe Acrobat - Quick View
 File Format: PDF/Adobe Acrobat - Quick View
File Format: PDF/Adobe Acrobat - Quick View
 File Format: Microsoft Powerpoint - Quick View
Rules for Naming Acids that Do Not Contain Oxygen in the Anion: Since all these acids have the same cation, H+, we don't need to name the cation. .
There is also a system of naming for organic (carbon-based) compounds. Naming Binary Compounds · Naming of Acids · Naming of Acids with Oxygen .
File Format: PDF/Adobe Acrobat - Quick View
Jump to Acids: Naming Acids. Simple covalent compounds that contain hydrogen, such as HCl, HBr, and HCN, often dissolve in water to produce acids. .
a. Any of a class of substances whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react .
Rules for Naming Acids, Bases and Salts. 1. Binary acids – acids that are made of only two elements. A. Prefix is always hydro .
The procedure for naming acids based on the anion name is shown. Examples given. How polyatomic ion containing species are named is also discussed. jcc cem .
File Format: Microsoft Powerpoint - Quick View
File Format: Microsoft Powerpoint - Quick View
Rules for Naming Acids that Do Not Contain Oxygen in the Anion: Since all these acids have the same cation, H+, we don't need to name the cation. .
There is also a system of naming for organic (carbon-based) compounds. Naming Binary Compounds · Naming of Acids · Naming of Acids with Oxygen .
File Format: PDF/Adobe Acrobat - Quick View
Jump to Acids: Naming Acids. Simple covalent compounds that contain hydrogen, such as HCl, HBr, and HCN, often dissolve in water to produce acids. .
a. Any of a class of substances whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react .
Rules for Naming Acids, Bases and Salts. 1. Binary acids – acids that are made of only two elements. A. Prefix is always hydro .
The procedure for naming acids based on the anion name is shown. Examples given. How polyatomic ion containing species are named is also discussed. jcc cem .
File Format: Microsoft Powerpoint - Quick View



 Aug 8, 2004 . The naming of inorganic acids does not follow the rules of the ionic compounds or covalent compounds. For example, HNO3 is called nitric .
Remember that the names of Arrhenius acids usually end in acid (hydrochloric acid, sulfuric acid, nitric acid ) and that their formulas fit one of two .
Aug 8, 2004 . The naming of inorganic acids does not follow the rules of the ionic compounds or covalent compounds. For example, HNO3 is called nitric .
Remember that the names of Arrhenius acids usually end in acid (hydrochloric acid, sulfuric acid, nitric acid ) and that their formulas fit one of two .


 File Format: PDF/Adobe Acrobat - Quick View
File Format: Microsoft Powerpoint - Quick View
Jump to Naming acids: If an acid is a binary compound, it is named as hydro[element]ic acid. If it contains a polyatomic ion, then it is named [ion .
Free video on naming acids. Naming acids eventually becomes second nature, but when naming acids, there are several guidelines that must be followed.
File Format: PDF/Adobe Acrobat - Quick View
File Format: Microsoft Powerpoint - Quick View
Jump to Naming acids: If an acid is a binary compound, it is named as hydro[element]ic acid. If it contains a polyatomic ion, then it is named [ion .
Free video on naming acids. Naming acids eventually becomes second nature, but when naming acids, there are several guidelines that must be followed.
 NAME THAT ACID! Rules for naming Acids: An acid is a compound that produces H+ ions in an aqueous (water) solution. Acids not containing a polyatomic ion .
2. a chemical compound that dissociates in solution, releasing hydrogen ions .
Nomenclature: Rules for Naming Simple Acids and Oxyacids. How do you recognize that something is an acid? The acids that we will be concerned with naming .
File Format: Microsoft Powerpoint - Quick View
File Format: PDF/Adobe Acrobat - Quick View
Naming Acids. 1. Some types of acids are formed by adding H+, (hydrogen ion), to a monatomic anion. For this type of acid, begin the name with "hydro", .
File Format: PDF/Adobe Acrobat - Quick View
Aug 18, 2010 . A summary on how to name acids. . Added to queue Naming Acids Iby utexascnsquest649 views · Thumbnail 7:58. Add to. Added to queue Acid .
First · Previous Next Last List of Slides · Notes · Text. Slide 9 of 9.
Naming Inorganic Compounds Chemistry-111 . .. If it is an acid, use the rules for naming acids. 5. If it is not an acid, use the rules for naming binary .
NAME THAT ACID! Rules for naming Acids: An acid is a compound that produces H+ ions in an aqueous (water) solution. Acids not containing a polyatomic ion .
2. a chemical compound that dissociates in solution, releasing hydrogen ions .
Nomenclature: Rules for Naming Simple Acids and Oxyacids. How do you recognize that something is an acid? The acids that we will be concerned with naming .
File Format: Microsoft Powerpoint - Quick View
File Format: PDF/Adobe Acrobat - Quick View
Naming Acids. 1. Some types of acids are formed by adding H+, (hydrogen ion), to a monatomic anion. For this type of acid, begin the name with "hydro", .
File Format: PDF/Adobe Acrobat - Quick View
Aug 18, 2010 . A summary on how to name acids. . Added to queue Naming Acids Iby utexascnsquest649 views · Thumbnail 7:58. Add to. Added to queue Acid .
First · Previous Next Last List of Slides · Notes · Text. Slide 9 of 9.
Naming Inorganic Compounds Chemistry-111 . .. If it is an acid, use the rules for naming acids. 5. If it is not an acid, use the rules for naming binary .
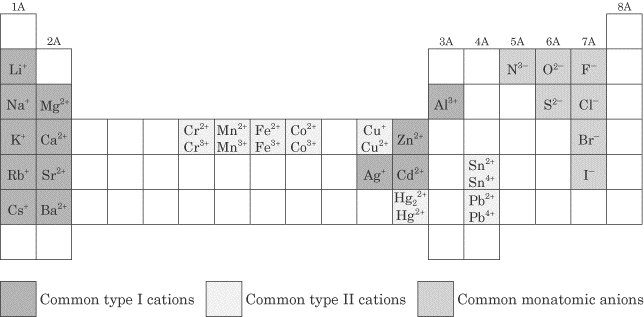 Sep 4, 2009 . Remove From Favorites Add To Favorites Naming AcidsProtected. page submenu . Binary Acids: Contain H and one other element .
III Naming Binary Acids. binary acid - consist of H+ and a nonmetal. EXAMPLE HCl H2S . H2S Hydrosulfuric acid. HI Hydroiodic acid. IV Naming Ternary Acids .
Sep 4, 2009 . Remove From Favorites Add To Favorites Naming AcidsProtected. page submenu . Binary Acids: Contain H and one other element .
III Naming Binary Acids. binary acid - consist of H+ and a nonmetal. EXAMPLE HCl H2S . H2S Hydrosulfuric acid. HI Hydroiodic acid. IV Naming Ternary Acids .
 Apr 6, 2010 . Vocabulary words for Use this set to master naming acids and bases . Includes studying games and tools such as flashcards.
File Format: Microsoft Word - Quick View
Sitemap
Apr 6, 2010 . Vocabulary words for Use this set to master naming acids and bases . Includes studying games and tools such as flashcards.
File Format: Microsoft Word - Quick View
Sitemap
|







































