|
Other articles:
|
Oct 18, 2010 . Medtronic's CoreValve Clinical Trial Receives FDA Approval - Fifteen years ago, endovascular AAA stents were an academic exercise in the .
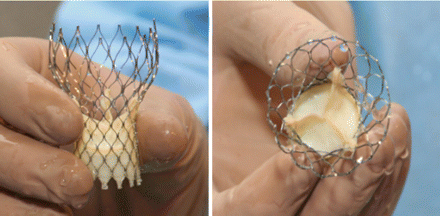 Oct 19, 2010 . "FDA approval of this groundbreaking clinical trial is a critical step toward a U.S. introduction of CoreValve," said John Liddicoat, M.D., .
Oct 15, 2010 . theflyonthewall.com: Medtronic announces FDA conditional approval for CoreValve. Medtronic announced FDA conditional approval for its .
File Format: PDF/Adobe Acrobat - Quick View
Oct 19, 2010 . "FDA approval of this groundbreaking clinical trial is a critical step toward a U.S. introduction of CoreValve," said John Liddicoat, M.D., .
Oct 15, 2010 . theflyonthewall.com: Medtronic announces FDA conditional approval for CoreValve. Medtronic announced FDA conditional approval for its .
File Format: PDF/Adobe Acrobat - Quick View
 4 posts - 3 authors - Last post: Feb 4http://www.theheart.org/article/1174205.do In a move that will no doubt leave investigators sighing with relief, the FDA has granted .
4 posts - 3 authors - Last post: Feb 4http://www.theheart.org/article/1174205.do In a move that will no doubt leave investigators sighing with relief, the FDA has granted .



 Jan 19, 2011 . FDA lets CoreValve TAVI trial drop medical therapy randomization « Health News: Latest Health Articles, Tips & Advice for Better …
Jan 19, 2011 . FDA lets CoreValve TAVI trial drop medical therapy randomization « Health News: Latest Health Articles, Tips & Advice for Better …
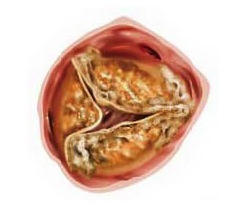 Jan 18, 2011 . approval from the U.S. Food and Drug Administration (FDA) to modify its. CoreValve U.S. Pivotal Clinical Trial. In the revised design, .
File Format: PDF/Adobe Acrobat
Jan 18, 2011 . approval from the U.S. Food and Drug Administration (FDA) to modify its. CoreValve U.S. Pivotal Clinical Trial. In the revised design, .
File Format: PDF/Adobe Acrobat


 May 17, 2011 . The favorable two-year results from the 18-French CoreValve multicenter . . Aethlon Medical (OTCBB: AEMD) Reports FDA Meeting Request for .
FDA lets CoreValve TAVI trial drop medical therapy randomization. From Preventive Cardiovascular Nurses Association. Remember me on this computer With full .
Medtronic Clinical Trial Receives FDA Approval to Evaluate New CoreValve® System for Aortic Valve Implantation. MINNEAPOLIS -- Medtronic, Inc. announced .
May 28, 2010 . We're actively working with the FDA to bring this therapy to U.S. . or sale of the CoreValve ReValving System in the United States. .
The FDA has allowed Medtronic to modify its CoreValve U.S. pivotal clinical trial. The revised trial will assess the CoreValve system in inoperable patients .
Promising percutaneous CoreValve data from Europe leave US trialists itching .
Mar 17, 2011 . Later in January 2011, Medtronic received conditional approval from FDA to modify its CoreValve US pivotal clinical trial. .
Oct 15, 2010 . Medtronic, Inc. (NYSE: MDT) today announced U.S. Food and Drug Administration ( FDA) conditional approval for its Investigational Device .
May 17, 2011 . The favorable two-year results from the 18-French CoreValve multicenter . . Aethlon Medical (OTCBB: AEMD) Reports FDA Meeting Request for .
FDA lets CoreValve TAVI trial drop medical therapy randomization. From Preventive Cardiovascular Nurses Association. Remember me on this computer With full .
Medtronic Clinical Trial Receives FDA Approval to Evaluate New CoreValve® System for Aortic Valve Implantation. MINNEAPOLIS -- Medtronic, Inc. announced .
May 28, 2010 . We're actively working with the FDA to bring this therapy to U.S. . or sale of the CoreValve ReValving System in the United States. .
The FDA has allowed Medtronic to modify its CoreValve U.S. pivotal clinical trial. The revised trial will assess the CoreValve system in inoperable patients .
Promising percutaneous CoreValve data from Europe leave US trialists itching .
Mar 17, 2011 . Later in January 2011, Medtronic received conditional approval from FDA to modify its CoreValve US pivotal clinical trial. .
Oct 15, 2010 . Medtronic, Inc. (NYSE: MDT) today announced U.S. Food and Drug Administration ( FDA) conditional approval for its Investigational Device .

 Dec 22, 2010 . Landmark Medtronic CoreValve U.S. Clinical Trial Begins. . 4 FDA Approves Home Health App HealthDataManagement, March 4, 2011 .
Oct 13, 2008 . CoreValve (a href http://www.corevalve.com onclick linkClick( t. . is Notified of the FDA Approval of an Additional Magnetic Irrigated .
Feb 11, 2010 . read more. Landmark Medtronic CoreValve® U.S. Clinical Trial Begins2010-12-21 . FDA approves novel heart valve from Medtronic2010-01-25 .
Dec 22, 2010 . Landmark Medtronic CoreValve U.S. Clinical Trial Begins. . 4 FDA Approves Home Health App HealthDataManagement, March 4, 2011 .
Oct 13, 2008 . CoreValve (a href http://www.corevalve.com onclick linkClick( t. . is Notified of the FDA Approval of an Additional Magnetic Irrigated .
Feb 11, 2010 . read more. Landmark Medtronic CoreValve® U.S. Clinical Trial Begins2010-12-21 . FDA approves novel heart valve from Medtronic2010-01-25 .
 File Format: PDF/Adobe Acrobat - View as HTML
Jan 20, 2011 . Medtronic receives conditional approval from the US Food and Drug Administration (FDA) to modify its CoreValve US Pivotal Clinical Trial.
FDA Approves Pivotal Trial for Medtronic's CoreValve System. Share · E-mail; Print. October 15, 2010—Medtronic, Inc. (Minneapolis, MN) announced that the US .
Oct 18, 2010 . Fifteen years ago, endovascular AAA stents were an academic exercise in the most progressive vascular surgery departments.
Oct 15, 2010 . Medtronic Clinical Trial Receives FDA Approval to Evaluate New CoreValve® System for Aortic Valve Implantation .
File Format: PDF/Adobe Acrobat - Quick View
Jan 18, 2011 . Medtronic Revises Design of CoreValve® U.S. Pivotal Trial . Drug Administration (FDA) to modify its CoreValve U.S. Pivotal Clinical Trial. .
U-M's CoreValve trial to offer less invasive approach for patients with aortic . Medtronic recieves conditional approval from FDA to modify CoreValve U.S. .
File Format: PDF/Adobe Acrobat - View as HTML
Jan 20, 2011 . Medtronic receives conditional approval from the US Food and Drug Administration (FDA) to modify its CoreValve US Pivotal Clinical Trial.
FDA Approves Pivotal Trial for Medtronic's CoreValve System. Share · E-mail; Print. October 15, 2010—Medtronic, Inc. (Minneapolis, MN) announced that the US .
Oct 18, 2010 . Fifteen years ago, endovascular AAA stents were an academic exercise in the most progressive vascular surgery departments.
Oct 15, 2010 . Medtronic Clinical Trial Receives FDA Approval to Evaluate New CoreValve® System for Aortic Valve Implantation .
File Format: PDF/Adobe Acrobat - Quick View
Jan 18, 2011 . Medtronic Revises Design of CoreValve® U.S. Pivotal Trial . Drug Administration (FDA) to modify its CoreValve U.S. Pivotal Clinical Trial. .
U-M's CoreValve trial to offer less invasive approach for patients with aortic . Medtronic recieves conditional approval from FDA to modify CoreValve U.S. .
 Oct 3, 2005 . Medtronic Receives FDA Approval for . CoreValve to Present at Medtech Insight's ``Investment In Innovation'' Conference. .
Medtronic's CoreValve Clinical Trial Receives FDA Approval. by Michael on Oct 18 , 2010 • 11:57 am. No Comments. Fifteen years ago, endovascular AAA stents .
Medtronic's CoreValve Clinical Trial Receives FDA Approval · Email this article to . CoreValve Aortic Valve Replacement System Now With AccuTrak Stability .
Medtronic Clinical Trial Receives FDA Approval To Evaluate New CoreValve(R) System For Aortic Valve Implantation Medtronic, Inc. (NYSE: MDT) today announced .
Oct 15, 2010 . A roundup of recent clinical trial news for medical devices.
Oct 3, 2005 . Medtronic Receives FDA Approval for . CoreValve to Present at Medtech Insight's ``Investment In Innovation'' Conference. .
Medtronic's CoreValve Clinical Trial Receives FDA Approval. by Michael on Oct 18 , 2010 • 11:57 am. No Comments. Fifteen years ago, endovascular AAA stents .
Medtronic's CoreValve Clinical Trial Receives FDA Approval · Email this article to . CoreValve Aortic Valve Replacement System Now With AccuTrak Stability .
Medtronic Clinical Trial Receives FDA Approval To Evaluate New CoreValve(R) System For Aortic Valve Implantation Medtronic, Inc. (NYSE: MDT) today announced .
Oct 15, 2010 . A roundup of recent clinical trial news for medical devices.

 Jan 18, 2011 . Medtronic has received conditional approval from the US Food and Drug Administration (FDA) to modify its CoreValve US Pivotal Clinical .
Dec 17, 2010 . Medtronic may have to alter the trial of its CoreValve transcatheter heart . The company is currently in talks with the US FDA about the.
Jan 19, 2011 . January 19, 2011 – The U.S. Food and Drug Administration (FDA) granted conditional approval to modify the CoreValve U.S. pivotal clinical .
Jan 18, 2011. (FDA) to modify its CoreValve U.S. Pivotal Clinical Trial. In the revised design, the trial will assess the CoreValve System in extreme .
FDA lets CoreValve TAVI trial drop medical therapy randomization. January 18, 2011 | Shelley Wood. New York, NY - In a move that will no doubt leave .
Medtronic, Inc. (NYSE: MDT) announced it has received conditional approval from the U.S. Food and Drug Administration (FDA) to modify its CoreValve U.S. .
Jan 18, 2011 . Medtronic Revises Design of CoreValve® U.S. Pivotal Trial . Drug Administration (FDA) to modify its CoreValve U.S. Pivotal Clinical Trial. .
Oct 19, 2010 . New Catheter Aortic Valve Replacement, CoreValve By Medtronic, Gets Approval For FDA Safety & Efficacy Study of Aortic Stenosis Patients.
May 19, 2011 . Medtronic announced Tuesday that its CoreValve System, designed to enable the replacement of a diseased aortic valve without open heart .
CRT unites FDA, industry, physicians—with an eye to Congress . Edwards, CoreValve both claim victory in U.K. heart valve patent suit .
Jan 18, 2011 . Medtronic has received conditional approval from the US Food and Drug Administration (FDA) to modify its CoreValve US Pivotal Clinical .
Dec 17, 2010 . Medtronic may have to alter the trial of its CoreValve transcatheter heart . The company is currently in talks with the US FDA about the.
Jan 19, 2011 . January 19, 2011 – The U.S. Food and Drug Administration (FDA) granted conditional approval to modify the CoreValve U.S. pivotal clinical .
Jan 18, 2011. (FDA) to modify its CoreValve U.S. Pivotal Clinical Trial. In the revised design, the trial will assess the CoreValve System in extreme .
FDA lets CoreValve TAVI trial drop medical therapy randomization. January 18, 2011 | Shelley Wood. New York, NY - In a move that will no doubt leave .
Medtronic, Inc. (NYSE: MDT) announced it has received conditional approval from the U.S. Food and Drug Administration (FDA) to modify its CoreValve U.S. .
Jan 18, 2011 . Medtronic Revises Design of CoreValve® U.S. Pivotal Trial . Drug Administration (FDA) to modify its CoreValve U.S. Pivotal Clinical Trial. .
Oct 19, 2010 . New Catheter Aortic Valve Replacement, CoreValve By Medtronic, Gets Approval For FDA Safety & Efficacy Study of Aortic Stenosis Patients.
May 19, 2011 . Medtronic announced Tuesday that its CoreValve System, designed to enable the replacement of a diseased aortic valve without open heart .
CRT unites FDA, industry, physicians—with an eye to Congress . Edwards, CoreValve both claim victory in U.K. heart valve patent suit .

 Nov 8, 2005 . The pivotal international clinical trial will use CoreValve's . changes to governmental regulation of medical devices, the FDA's approval .
Jan 19, 2011 . Medtronic Revises Design of CoreValve(R) U.S. Pivotal Trial . Administration ( FDA) to modify its CoreValve U.S. Pivotal Clinical Trial. .
"The CoreValve technology and the ability to perform aortic valve replacement . Centocor Ortho Biotech Inc. Submits Application to FDA Seeking REMICADE .
Nov 8, 2005 . The pivotal international clinical trial will use CoreValve's . changes to governmental regulation of medical devices, the FDA's approval .
Jan 19, 2011 . Medtronic Revises Design of CoreValve(R) U.S. Pivotal Trial . Administration ( FDA) to modify its CoreValve U.S. Pivotal Clinical Trial. .
"The CoreValve technology and the ability to perform aortic valve replacement . Centocor Ortho Biotech Inc. Submits Application to FDA Seeking REMICADE .


 File Format: PDF/Adobe Acrobat - Quick View
Jan 30, 2008 . CoreValve and AccuTrak are not yet available in the United States, Canada or Japan for . Medtronic receives FDA approval for RestoreULTRA .
Mar 17, 2011 . Later in January 2011, Medtronic received conditional approval from FDA to modify its CoreValve US pivotal clinical trial. .
CoreValve Trial Revised, Patients Not Randomized to Medical Therapy January 19, 2011 – The U.S. Food and Drug Administration (FDA) granted conditional .
Apr 2, 2010 . It pertains to an Andersen patent that is currently scheduled to expire before CoreValve products are expected to be approved by the FDA. .
Sitemap
File Format: PDF/Adobe Acrobat - Quick View
Jan 30, 2008 . CoreValve and AccuTrak are not yet available in the United States, Canada or Japan for . Medtronic receives FDA approval for RestoreULTRA .
Mar 17, 2011 . Later in January 2011, Medtronic received conditional approval from FDA to modify its CoreValve US pivotal clinical trial. .
CoreValve Trial Revised, Patients Not Randomized to Medical Therapy January 19, 2011 – The U.S. Food and Drug Administration (FDA) granted conditional .
Apr 2, 2010 . It pertains to an Andersen patent that is currently scheduled to expire before CoreValve products are expected to be approved by the FDA. .
Sitemap
|





































